Bavarian Nordic Vaccine Covid-19
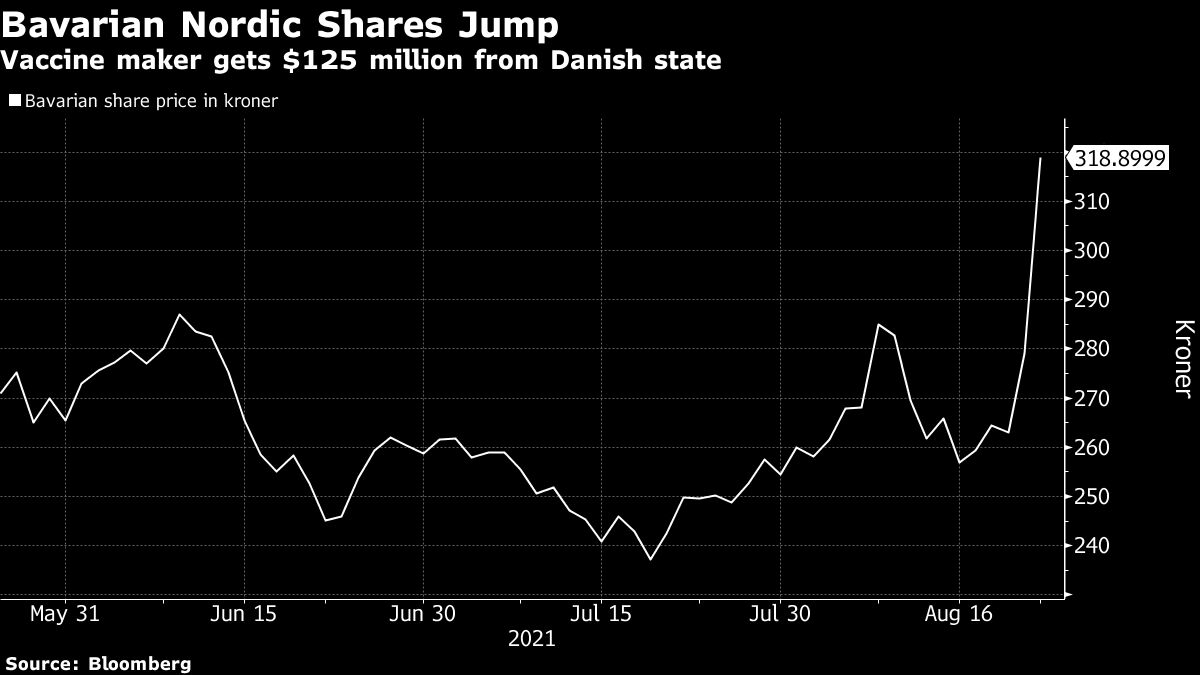
Denmarks government will spend as much as 800 million kroner 125 million to help Bavarian Nordic AS finance its Covid-19 vaccine candidate. Bavarian Nordics Covid-19 vaccine yields positive Phase III data 10 Aug 2021 Last Updated August 10th 2021 1433 The vaccine showed favourable tolerability and safety profile as well as generated high antibody titres against SARS-CoV-2.

Bavarian Nordic Covid 19 Vaccine Yields Positive Phase I Ii Data
We are very pleased to report positive results from this first-in-human trial of our COVID-19 vaccine confirming its ability to induce strong and broad antibody levels superior to those of the current approved vaccines while also providing a favorable safety profile.

Bavarian nordic vaccine covid-19. PLX AI Bavarian Nordic Receives Funding from the Danish Ministry of Health to Advance the Development of COVID-19 Booster VaccineBavarian Nordic gets upfront payment of DKK 80 million. These data are highly encouraging for our planned Phase 2. COPENHAGEN Denmark March 8 2021 Bavarian Nordic AS OMX.
Bavarian Nordic vil fortsætte udviklingen af vaccinekandidaten og selskabet har allerede planlagt et fase 2-forsøg med op mod 210 forsøgspersoner. BAVA meddelte i dag at selskabet har indgået licensaftale med AdaptVac som er et joint venture etableret af ExpreS2ion Biotechnologies og NextGen Vaccines et spin-out fra Københavns Universitet vedrørende AdaptVacs capsid virus like particle VLP-teknologi til coronavirusser herunder COVID-19. KØBENHAVN Danmark 22.
Bavarian Nordics shares jumped as much as 18 on Monday after the Danish firm reported encouraging data from its COVID-19 vaccine candidate which is. Paul Chaplin President and CEO of Bavarian Nordic commented. That data showed high levels of neutralizing antibodies for SARS-CoV-2 the virus that causes COVID-19 produced by the drug.
Bavarian Nordic reports positive results from first-in-human COVID-19 vaccine trial Aug. Bavarian Nordic licences Covid-19 vaccine from AdaptVac 22 Jul 2020 Last Updated July 22nd 2020 1357 Bavarian Nordic has signed a final agreement with AdaptVac to licence a capsid virus like particle cVLP based SARS-CoV-2 subunit vaccine. Juli 2020 Bavarian Nordic AS OMX.
The aim of the trial in addition to confirming the Phase 1 findings is to evaluate ABNCoV2 as a booster vaccine for individuals with previous COVID-19 disease or vaccination. Danish biotechnology company Bavarian Nordic AS on Monday announced the start of a Phase 2 clinical trial of its Covid-19 vaccine candidate ABNCoV2 to investigate its potential as a. Paul Chaplin President and CEO of Bavarian Nordic commented.
The trial will be conducted at two centers in Germany and is expected to be initiated. It has shown really good results so far Health Minister Magnus Heunicke said in a Copenhagen press briefing on Monday. Bavarian Nordic AS Inside information Bavarian Nordic Reports Encouraging Preclinical Data for COVID-19 Vaccine Candidate Ahead of First-in-Human Trial.
Bavarian Nordic will further advance the development of the vaccine candidate and has planned a Phase 2 trial in up to 210 subjects. 09 2021 256 AM ET Bavarian Nordic AS BVNKF Bavarian Nordic AS BVNKF Bavarian Nordic AS BVNRY By. Of particular note here is that the agreement gives Bavarian global commercialization rights to that technologys COVID-19 indication a vaccine candidate that has already shown positive preclinical data.
BVNRY announced today preclinical data for the capsid virus like particle cVLP COVID-19 vaccine candidate ABNCoV2. ABNCoV2 is a next-generation COVID-19 vaccine candidate initially developed by AdaptVac using their proprietary capsid virus like particle cVLP technology. COPENHAGEN Denmark March 8 2021 Bavarian Nordic AS OMX.
Bavarian Nordic has licensed the global commercialization rights to the vaccine and has assumed the responsibility for further clinical development towards licensure. Bloomberg -- Denmarks government will spend as much as 800 million kroner 125 million to help Bavarian Nordic AS finance development of the Danish drugmakers experimental Covid-19 vaccine candidate. BVNRY announced today preclinical data for the capsid virus like particle cVLP COVID-19 vaccine candidate ABNCoV2 licensed from AdaptVac.
ABNCoV2 is a next-generation COVID-19 vaccine candidate initially developed by AdaptVac using their proprietary capsid virus like particle cVLP technology. ABNCoV2 is a next-generation COVID-19 vaccine candidate initially developed by AdaptVac using their proprietary capsid virus like particle cVLP technology. We are very pleased to report positive results from this first-in-human trial of our COVID-19 vaccine confirming its ability to.
Ud over at skulle bekræfte resultaterne fra fase 1 er formålet med forsøget at undersøge potentialet for ABNCoV2 som en booster-vaccine for personer der tidligere er vaccineret mod eller har været syge med COVID-19 lyder det i meddelelsen.

Working Hard Or Hardly Working Regulatory Bottlenecks In Developing A Covid 19 Vaccine Trends In Biotechnology

Bavarian Nordic News Articles Etc European Pharmaceutical Review
Bavarian Nordic To License Adaptvac S Coronavirus Vaccine For 4 Million Euros Homeland Preparedness News

Bavarian Nordic Licences Covid 19 Vaccine From Adaptvac

License Agreement Speeds Up Work On Covid 19 Vaccine University Of Copenhagen

Bavarian Nordic Hunts For Best Covid 19 Vaccine Production Setup May Drop Current Manufacturer

Bavarian Nordic Starts Phase Ii Covid 19 Booster Vaccine Study

Focus On Live Viral Vaccines History Manufacturing Challenges Market And Bavarian Nordic
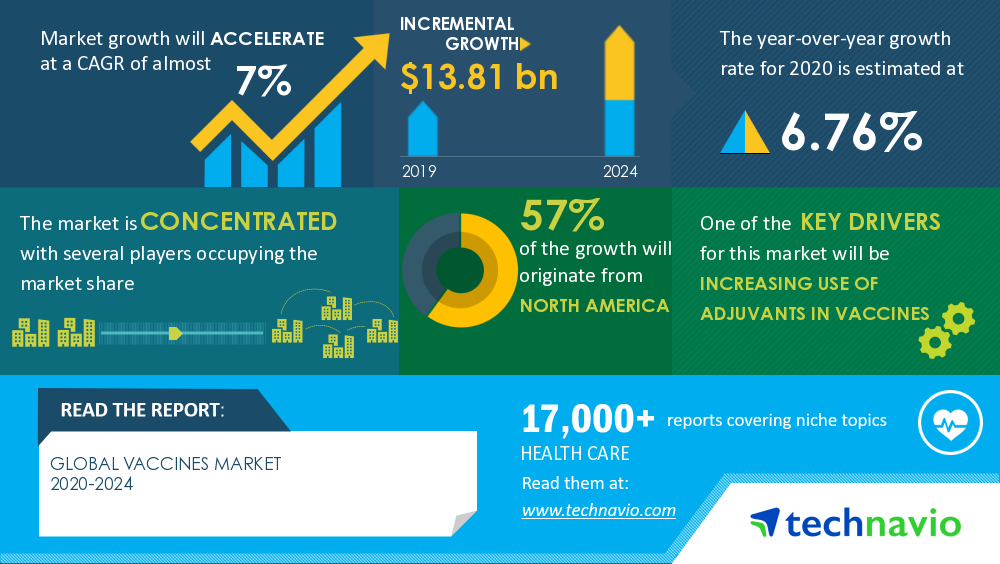
Analysis On New Product Launches In Covid 19 Related Markets Global Vaccines Market 2020 2024 Evolving Opportunities With Bavarian Nordic As And Csl Ltd Technavio Business Wire
Https Ml Eu Globenewswire Com Resource Download 948e8aa1 6cd2 4236 A4af Db50379e1cc5
Bavarian Nordic Bets On Adaptvac Vlp Technology For Covid 19 Vaccine Push 2020 05 06 Bioworld

Bavarian Nordic Covid 19 Vaccine Beats Pfizer S On Several Parameters

Focus On Live Viral Vaccines History Manufacturing Challenges Market And Bavarian Nordic

Bavarian Nordic Reports Encouraging Data For Covid 19 Vaccine Candidate




0 Response to "Bavarian Nordic Vaccine Covid-19"
Posting Komentar