Bavarian Nordic Vaccine Covid
Bavarian Nordic vil fortsætte udviklingen af vaccinekandidaten og selskabet har allerede planlagt et fase 2-forsøg med op mod 210 forsøgspersoner. 09082021 - PLX AI Bavarian Nordic shares rose more than 4 after announcing positive results in a Covid-19 vaccine trial.

Vaccines Market Analysis Highlights The Impact Of Covid 19 2020 2024 Increasing Use Of Adjuvants In Vaccines To Boost The Market Growth Technavio
Denmarks government will spend as much as 800 million kroner 125 million to help Bavarian Nordic AS finance its Covid-19 vaccine candidate.
Bavarian nordic vaccine covid. These data are highly encouraging for our planned Phase 2. Vaccine was well tolerated across all dose. ABNCoV2 has shown to be highly immunogenic in relevant.
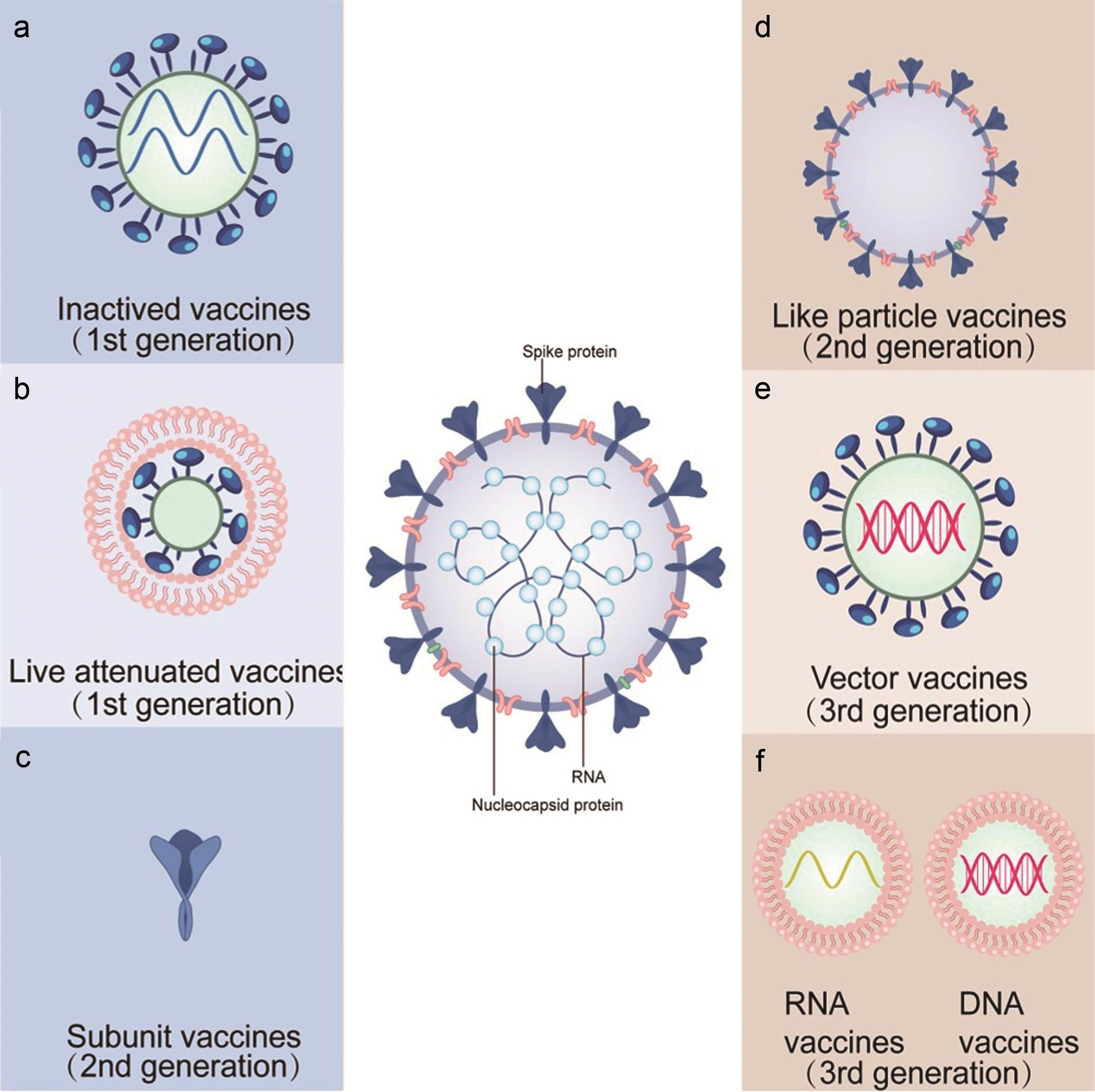
The company is. Bavarian Nordic has signed a final agreement with AdaptVac to licence a capsid virus like particle cVLP based SARS-CoV-2 subunit vaccine. That data showed high levels of neutralizing antibodies for SARS-CoV-2 the virus that causes COVID-19 produced by the drug.
Subjects were enrolled at Radboud University Medical Centre in the Netherlands. Bavarian Nordic AS Inside information Bavarian Nordic Reports Encouraging Preclinical Data for COVID-19 Vaccine Candidate Ahead of First-in-Human Trial. Paul Chaplin President and CEO of Bavarian Nordic commented.
Its first clinical study is scheduled for the fourth quarter of this. Det skriver Sundhedsministeriet i en pressemeddelelse. Bloomberg the Company Its Products The Company its Products Bloomberg Terminal Demo Request Bloomberg Anywhere Remote Login Bloomberg Anywhere Login Bloomberg Customer Support Customer Support.
We are very pleased to report positive results from this first-in-human trial of our COVID-19 vaccine confirming its ability to. Biotechnology company Bavarian Nordic AS may sell its Covid-19 vaccine candidate if it cant raise the money needed to carry out trials on humans. Led by the PREVENT-nCoV consortium the trial involved 45 healthy adults with no previous SARS-CoV-2 infection.
ABNCoV2 is a next-generation COVID-19 vaccine candidate initially developed by AdaptVac using their proprietary capsid virus like particle cVLP technology. Paul Chaplin President and CEO of Bavarian Nordic commented. PLX AI - Bavarian Nordic shares rose 3 at the open after the company started a phase 2 trial for its Covid-19 booster vaccine.
We are very pleased to report positive results from this first-in-human trial of our COVID-19 vaccine confirming its ability to induce strong and broad antibody levels superior to those of the current approved vaccines while also providing a favorable safety profile. AdaptVac plans to launch a trial of the Covid-19 vaccine before the end of this year. Ud over at skulle bekræfte resultaterne fra fase 1 er formålet med forsøget at undersøge potentialet for ABNCoV2 som en booster-vaccine for personer der tidligere er vaccineret mod eller har været syge med COVID-19 lyder det i meddelelsen.
Danish biotechnology company Bavarian Nordic AS on Monday announced the start of a Phase 2 clinical trial of its Covid-19 vaccine candidate ABNCoV2 to investigate its potential as a. Bavarian Nordics shares jumped as much as 18 on Monday after the Danish firm reported encouraging data from its COVID-19 vaccine candidate which is. ABNCoV2 is a next-generation COVID-19 vaccine candidate initially developed by AdaptVac using their proprietary capsid virus like particle.
Bavarian Nordic has announced positive preliminary data from the first-in-human Phase III dose-escalation clinical trial of its Covid-19 vaccine ABNCoV2. Regeringen har besluttet at støtte virksomheden Bavarian Nordic med 800 millioner kroner til udvikling af en dansk vaccine mod covid-19. It has shown really good results so far Health Minister Magnus Heunicke said in a Copenhagen press briefing on Monday.
Bavarian Nordic has licensed the global commercialization rights to the vaccine and has assumed the responsibility for further clinical development towards licensure. Of particular note here is that the agreement gives Bavarian global commercialization rights to that technologys COVID-19 indication a vaccine candidate that has already shown positive preclinical data. COPENHAGEN Denmark March 8 2021 Bavarian Nordic AS OMX.
Bloomberg -- Denmarks government will spend as much as 800 million kroner 125 million to help Bavarian Nordic AS finance development of the Danish drugmakers experimental Covid-19 vaccine candidate. PLX AI Bavarian Nordic Initiates Phase 2 Clinical Trial of COVID-19 Booster Vaccine. The vaccine is expected to be approved early next.
Phase 2 trial will investigate vaccines ability to boost existing immunity from prior COVID-19. Det sker efter at EU-Kommissionen har godkendt at Danmark kan. BVNRY announced today preclinical data for the capsid virus like particle cVLP COVID-19 vaccine candidate ABNCoV2 licensed from AdaptVac.

Molekylaer Velcro Er Noglen Bag Staerkt Immunrespons I Dansk Covid 19 Vaccine Dagens Medicin

Dk Stotteforanstaltning Til Coronavirusrelaterede Forsknings Og Udviklingsaktiviteter I Bavarian Nordic Godkendt

Bavarian Nordic Praesentation Af Arsrapporten Og Opdatering Pa Covid 10 Vaccine Youtube

Coronavirus Digest Uk Hits New Covid Infection Record News Dw 28 12 2020
Mva Bn Smallpox Vaccine Bavarian Nordic
J J Vaccine Recipients Seek Mrna Booster Without Cdc S Blessing Bloomberg

Annual Report 2020 Bavarian Nordic

Bavarian Nordic Investor Relations

Brazil S Halting Of China Vaccine Baffles Local Researchers Bloomberg
Barda Expands Contracts With Bavarian Nordic On Smallpox Vaccine Homeland Preparedness News

Merck Bets On Viral Vector Vaccine Platforms Plus Oral Antiviral In Three Way Covid 19 Push 2020 05 26 Bioworld




0 Response to "Bavarian Nordic Vaccine Covid"
Posting Komentar